About
The Biosensors for bioengineering group is a senior group under ICREA’s Tenure Track scheme.
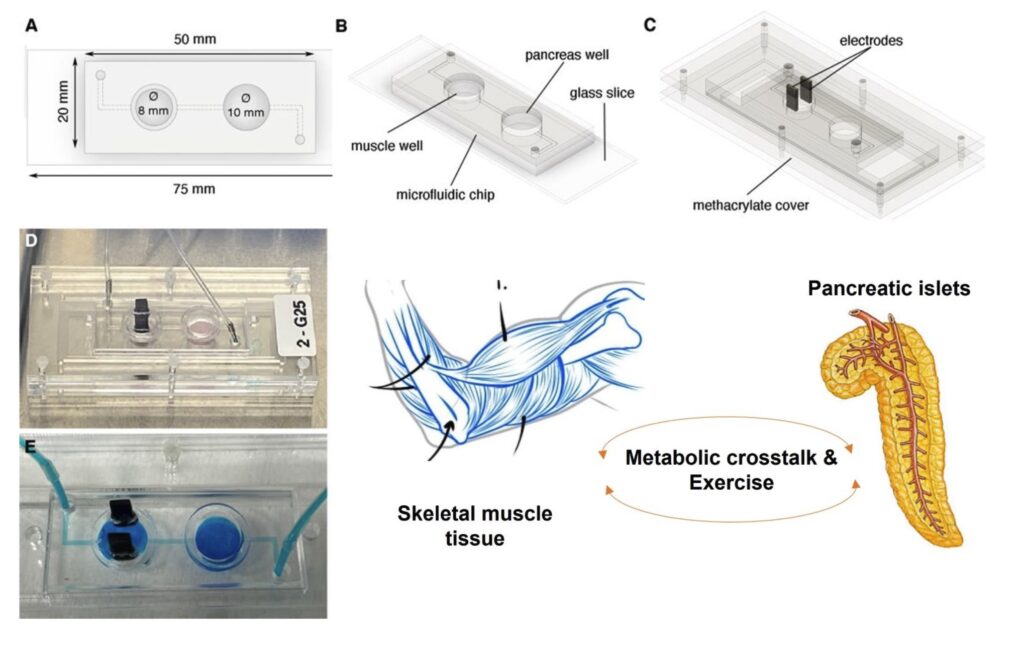
Organs-on-a-chip (OOC) refers to a technology that involves creating microscale devices that mimic the structure and function of human organs. These “chips” are typically composed of living cells arranged on a microfluidic platform, allowing researchers to simulate the complex interactions and physiological responses within a specific organ.
The goal of Organs-on-a-chip (OOC) technology is to provide a more accurate and representative model of human organs compared to traditional in vitro cell cultures or animal testing. By replicating the microenvironment of organs and incorporating various cell types, organs-on-a-chip can help researchers study the effects of drugs, toxins, and diseases in a more realistic and controlled manner.
Each organ-on-a-chip device is designed to replicate the unique characteristics of a particular organ, such as the liver, pancreas or skeletal muscle. These miniature systems enable researchers to observe and analyze how different substances and conditions affect cellular behavior, tissue function, and overall organ responses. The technology holds promise for drug development, disease modeling, and toxicology studies, offering a more ethical and efficient alternative to traditional methods.
Our research on OOC development has clear goals. We want OOC platforms to be easy to use and more automation to set up cell cultures. This will help more people use them, making experiments quicker and more reliable.
We’re working on creating a simple platform for growing microtissues in 3D. This makes OOC research easier to use in the real world, moving from lab tests to practical applications. We also want to improve the user experience by making OOC platforms more friendly, compatible, and ready for use.
As OOC research moves from labs to real-world use, we want to help users deal with biological challenges. We’re developing an easy-to-use 3D tissue platform and a simple bioreactor that works with sensing technology. This helps users focus on solving biological problems, validating models, and finding potential medicines. We’re also adding sensors to make the bioreactor even more effective.
Our efforts make it easier for researchers to study how organs interact. Our second goal focuses on studying more complex disease models. This helps us understand diseases better and find ways to treat them. We believe that OOCs help in three main ways: understanding diseases, making better medicines faster, and supporting personalized research using cells from individual patients. OOCs can be a solution for studying rare diseases where other methods are not available.
Our third goal is to standardize OOC platforms, making them work together better. This involves creating common rules and standards for everyone to follow. This makes collaboration and sharing information easier.
Finally, our lab is working towards making OOCs suitable for high-throughput screening. This means making them simpler and adding good models for studying diseases. In short, we’re making OOC development more user-friendly, accessible, and technologically advanced. This simplification helps in biological research and finding new medical solutions.
Staff
Javier Ramón Azcón
Projects
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| Development of a “Muscle-on-a-Chip” (MoC) platform for the preclinical evaluation of potential therapies for Duchenne muscular dystrophy (2020-2022) | DUCHENNE ESPAÑA, IV Convocatoria Ayudas a Proyectos de Investigación | Juanma Fernandez |
| BLAD · BioLiver Assist Device (2020-2021) | AGAUR, Ajuts per a projectes innovadors amb potencial d’incorporació al sector productiu – LLAVOR | Javier Ramón |
| INNOTEC- Javier Ramon- Naturfiltr (2021-2023) | TECNIO | Javier Ramón |
| ASITOC Atomic-Sensor-Integrated Tissue-On-a-Chip: optically detected biomagnetism to understand muscular diseases (2021-2022) | BIST_Barcelona Institute of Science and Technology | Juanma Fernandez |
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| DAMOC · ‘Diabetes Approach by Multi-Organ-on-a-Chip’ (2017-2022) | ERC | Javier Ramón |
| BLOC · Benchtop NMR for Lab-on-Chip (2020-2022) | European Comission FET-Open | Javier Ramón |
| PRIVATELY FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| Tatami · Therapeutic targeting of MBNL microRNAs as innovative treatments for myotonic dystrophy (2019-2022) | Fundació bancaria “La Caixa” | Javier Ramón |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| Programa Faster Future 2020: COVID-19 (2021) | Fundraising | Javier Ramón |
| INDUCT · Fabrication of a biomimetic in vitro model of the intestinal tube muscle wall: smooth muscle-on-a-chip (2018-2020) | MINECO | Javier Ramón |
Publications
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
Equipment
Micro and nanofabrication techniques:
- 3D microstructures on hydrogel materials
- Mini-bioreactor for 3D cell culture
- Microelectrodes fabrication
- Synthesis and chemical modification of polymers and surfaces
- Dielectrophoretic cells and micro particles manipulation
Characterization techniques:
- Optical Microscopes (white light/epifluorescence)
- Electrochemical techniques (Potentiometric/Amperometric/Impedance spectroscopy)
- Immunosensing techniques (Fluorescence ELISA/Colorimetric ELISA/magneto ELISA)
Equipment:
- Microfluidic systems (High precision syringe pumps/Peristaltic pumps/Micro valves)
- Biological safety cabinet (class II)
- Epifluorescence microscope for live-cell imaging
- Pulsar – a high-resolution, 60MHz benchtop NMR spectrometer from Oxford Instruments
Access to the Nanotechnology Platform (IBEC Core Facilities): equipment for hot embossing lithography, polymer processing and photolithography, chemical wet etching, e-beam evaporation and surface characterization (TOF-SIMS)
Access to the Scientific and Technological Centers (University of Barcelona): equipment for surface analysis (XPS, AFM, XRD), organic structures characterization (NMR) and microscopy techniques (SEM, TEM, confocal)
Collaborations
We collaborated closely with Professor Ruben Artero from Instituto de Investigaciones Clínicas de Valencia (INCLIVA) and medical doctor Vilchez from Hospital de la Fe (Valencia). We develop muscle-on-a-chip devices using 3D tissue cultures and biosensors. During my career, I established national and international collaborations with other researchers, clinicians, and companies. This is reflected by the fact that I attracted competitive funding awarded by the prestigious entity Medical Research Council (UK), focused on studying Duchenne’s rare disease. I also collaborate on projects with more clinical groups and hospitals, e.g., Hospital de Sant Pau (Barcelona). With senior professor Eduard Gallardo’s group, we are developing human microtissues to study the myasthenia gravis neuromuscular rare disease.
Following the translational nature of my research, I recently became the entrepreneurial scientist of a valorisation project financed by Producte Call (AGAUR) to bring to the market plasmonic biosensors for Myasthenia Gravis diagnosis. I actively collaborate with patient associations such as “Duchenne Parent Project ” and “Asociación Conquistando Escalones,” and with national and international companies such as Arthex biotech, SOM biotech, BI/OND (The Netherlands), and BioEmTech (Greece). I have also established contacts with the industry to develop new technology with a high impact on clinical diagnosis and drug development. Specifically, we collaborate with Grifols (Spain), Multivawe (Switzerland), Oxford Instrument (UK) and NovoNordisk (Denmark). This last collaboration aims to develop new biomaterials for cell therapies. I have also established contacts with the industry to develop new technology with a high impact on clinical diagnosis and drug development, specifically collaborating with Multiwave (Switzerland) and Oxford Instrument (United Kingdom). I am also co-founder of a spin-off company, Vitala.
News

IBEC secures competitive funding to drive forward validation testing for three innovative health projects
IBEC has been awarded three grants in response to the call for proposals from the Strategic Plan for Health Research and Innovation (PERIS). These projects aim to validate hyperspectral technology for improving assisted reproduction, using bioprinted cell spheroids to combat type 1 diabetes and developing light-activated drugs to restore vision in patients with retinal degeneration.

The IBEC and West China Hospital hold their third joint conference on precision medicine in Barcelona
Barcelona hosted the third IBEC-WCH Precision Medicine Conference this week, an event that further strengthened the strategic alliance between the Institute for Bioengineering of Catalonia (IBEC) and the West China Hospital of Sichuan University (WCHSU). The Chinese delegation visited Spain from 26 to 29 November, taking part in a busy schedule of scientific and institutional activities and exchanges between the two centres.

Fibrosens Project receives funds from AFM-Telethon to develop sensor devices for muscular dystrophy
IBEC researcher Juanma Fernández recently has received funding from the French AFM-Telethon to carry out the project “Monitoring of fibrotic processes in 3D skeletal muscle co-cultures for Muscular Dystrophies using plasmonic biosensors”. The objective is to develop multiplexing sensor devices to achieve online, real-time sensing capability to monitor fibrosis markers and evaluate drug response in muscular dystrophy in vitro models.

IBEC science reaches thousands at the 43rd Comic Barcelona
The Institute for Bioengineering of Catalonia (IBEC) was a major presence at the 43rd Comic Barcelona last week. Through an exhibition on everyday science, talks and informative workshops, IBEC was able to bring its cutting-edge research to an audience of all ages.

IBEC and SJD Barcelona Children’s Hospital strengthen their collaboration with a day of translational innovation
The Institute for Bioengineering of Catalonia and the Sant Joan de Déu Barcelona Children’s Hospital have held a joint conference to strengthen collaboration in bioengineering and translational medicine. The event, held this morning at the IBEC, highlighted innovative projects, presented a joint PhD programme and encouraged the exchange of ideas between researchers from both institutions.

IBEC and BST strengthen ties with Translational Collaboration Day
IBEC and the Blood and Tissue Bank of Catalonia (BST) held a day to explore new collaborations in bioengineering and translational medicine. The meeting, held yesterday at IBEC, highlighted innovative projects, presented a joint PhD programme and strengthened the link between biomedical research and clinical applications.

Organ-on-Chips to reduce animal testing
IBEC is one of the partners in the ambitious European project UNLOOC, a public-private collaboration involving 51 organisations from 10 countries with a budget of €68 million. The consortium aims to develop Organ-on-Chips technologies to reduce the use of animals in drug development and testing, and to improve the accuracy and personalisation of medical treatments.

IBEC and West China Hospital strengthen collaboration in precision medicine
The second IBEC-WCH Precision Medicine Conference took place last week in Chengdu, China. This is a partnership between the Institute for Bioengineering of Catalonia (IBEC) and the West China Hospital (WCH) of Sichuan University, which aims to strengthen scientific collaboration between the two countries.
Two projects with IBEC participation selected in the MSCA call for PhD networks
IBEC will coordinate SPM4.0 and participate as a partner in ENTRY-DM, two of the projects selected in the 2023 call for PhD networks within the Marie Skłodowska-Curie Actions (MSCA). Thanks to these two projects, IBEC will add three new PhD students to its staff.

IBEC to Develop Organs-on-a-Chip in Three Pathfinder Projects
BuonMarrow, OMICSENS, and PHOENIX-OoC are the three projects in which IBEC’s Biosensors for Bioengineering Group will apply its extensive knowledge in the field of biosensors and organs-on-a-chip. The projects, which will be developed with funding from the European Innovation Council’s prestigious Pathfinder Open program, promise to enhance cancer treatments and foster innovation in diagnostics.
Jobs
Postdoctoral Researcher at the Biosensors for Bioengineering Research Group
Ref: JR-PR // Deadline:18/02/2026
Research Assistant at the Biosensors for Bioengineering Research Group
Ref: RA-JR // Deadline: 05/12/2025
Research Assistant at the Biosensors for Bioengineering Research Group
Ref: JR_RA // Deadline: 24/11/2025
Laboratory Assistant position at the Biosensors for Bioengineering Research Group
Ref: LA-JR // Deadline: 22/10/2025
Laboratory Assistant at the Biosensors for Bioengineering Research Group
Ref: LA-JR // Deadline: 15/10/2025
Researcher Training position at the Biosensors for Bioengineering Research Group
Ref: RT_JR // Deadline: 15/08/2025
Researcher in Training at the Biosensors for Bioengineering Research Group
Ref: RT_JR //Deadline: 07/02/2025
Laboratory Technician at the Biosensors and Bioengineering Research Group
Ref: LT_jr//Deadline: 18/11/2024
Research Assistant position at the Biosensors for Bioengineering Research Group
Ref: RA- JR // Deadline: 23/09/2024
Research Assistant position at the Biosensors for Bioengineering Research Group
Ref: RA- JR-2 // Deadline: 21/08/2024


 ibecbarcelona.eu
ibecbarcelona.eu