
Javier Ramón Azcón
About
Drug discovery pathway relies heavily on in vivo animal models and in vitro cell mediums. In the case of animal models we have not only some ethical problems but also the ability to extrapolate data to human conditions is limited and in vitro platforms often do not simulate the complex cell–cell and cell–matrix interactions crucial for regulating cell behaviour.
The Biosensors for Bioengineering group is focused in a new line of research that has become of extreme importance in the last years. The idea is to integrate biosensor technology and nanotechnology with stem cell research and with tissue engineering. Engineered tissues are integrated with biosensing technology to obtain microdevices for detecting cellular responses to external stimuli, monitoring the quality of the microenvironment (e.g., metabolites, nutrients), and supporting diverse cellular requirements. This research on 3D-functional engineered tissues is expected to develop knowledge of tissue construction and their functions and relation with some human diseases. Integration of fully functional tissues with microscale biosensor technology allowed us to obtain “organs-on-a-chip”. These chips could be used in pharmaceutical assays and could be a step toward the ultimate goal of producing in vitro drug testing systems crucial to the medicine and pharmaceutical industry.
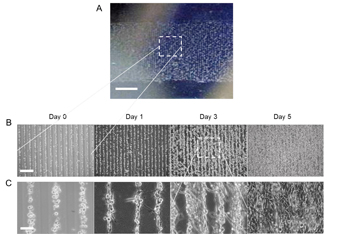
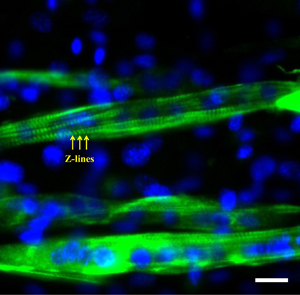
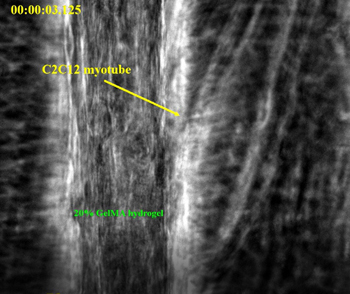
Right: Picture of aligned C2C12 muscle cells within hydrogel as obtained by the dielectrophoresis (DEP) technique using 50 µm electrode 50 µm gap device (A). Phase contrast images of the aligned C2C12 muscle cells within hydrogel at different culture times (B and C). Scale bar shows 0.25 cm, 400 μm, and 50 μm in A, B, and C, respectively.
News/Jobs
IBEC to launch Faster Future, a new fundraising initiative, on Giving Tuesday
22/11/17
Next week IBEC will launch Faster Future, a new crowdfunding initiative that aims to help accelerate research projects that are close to tackling major challenges in health.
Finding out about diabetes approaches at IBEC’s Setmana de la Ciencia event
16/11/17
Yesterday IBEC group leader Javer Ramon presented his research on developing organ-on-a-chip to study diabetes in IBEC’s public event forming part of this year’s Setmana de la Ciència.
IBEC researcher’s ERC project highlighted in Madrid exhibition
28/03/17
This weekend Javier Ramon’s European Research Council-funded project, DAMOC, was one of eight highlighted in a special exhibition in Madrid to mark the ERC’s tenth anniversary.
ERC funding for new diabetes approach at IBEC
04/10/16
IBEC’s Dr. Javier Ramón is one of just six researchers in Catalonia to have been awarded a 2016 Starting Grant by the European Research Council (ERC).
Projects
EU-funded projects
| ‘Diabetes Approach by Multi-Organ-on-a-Chip’ (DAMOC) | ERC | Javier Ramón |
Publications
(See full publication list in ORCID)
[br]
Equipment
Micro and nanofabrication techniques:
- 3D microstructures on hydrogel materials
- Mini-bioreactor for 3D cell culture
- Microelectrodes fabrication
- Synthesis and chemical modification of polymers and surfaces
- Dielectrophoretic cells and micro particles manipulation
Characterization techniques:
- Optical Microscopes (white light/epifluorescence)
- Electrochemical techniques (Potentiometric/Amperometric/Impedance spectroscopy)
- Immunosensing techniques (Fluorescence ELISA/Colorimetric ELISA/magneto ELISA)
Equipment:
- Microfluidic systems (High precision syringe pumps/Peristaltic pumps/Micro valves)
- Biological safety cabinet (class II)
- Epifluorescence microscope for live-cell imaging
Access to the Nanotechnology Platform (IBEC Core Facilities): equipment for hot embossing lithography, polymer processing and photolithography, chemical wet etching, e-beam evaporation and surface characterization (TOF-SIMS)
Access to the Scientific and Technological Centers (University of Barcelona): equipment for surface analysis (XPS, AFM, XRD), organic structures characterization (NMR) and microscopy techniques (SEM, TEM, confocal)
Collaborations
- Prof. Josep Samitier
IBEC - Dr. Elena Martinez
IBEC - Dr. Anna Novials
Institut d’Investigacions Biomediques August Pi i Sunyer (IDIBAPS) - Dr. Ramon Gomís
Institut d’Investigacions Biomediques August Pi i Sunyer (IDIBAPS) - Dr. Eduard Montanya
The Bellvitge Biomedical Research Institute (IDIBELL) - Prof. Enric Bertran
Physics and Engineering of Amorphous Materials and Nanostructures (FEMAN), Department of Applied Physics, University of Barcelona


 ibecbarcelona.eu
ibecbarcelona.eu