ABOUT
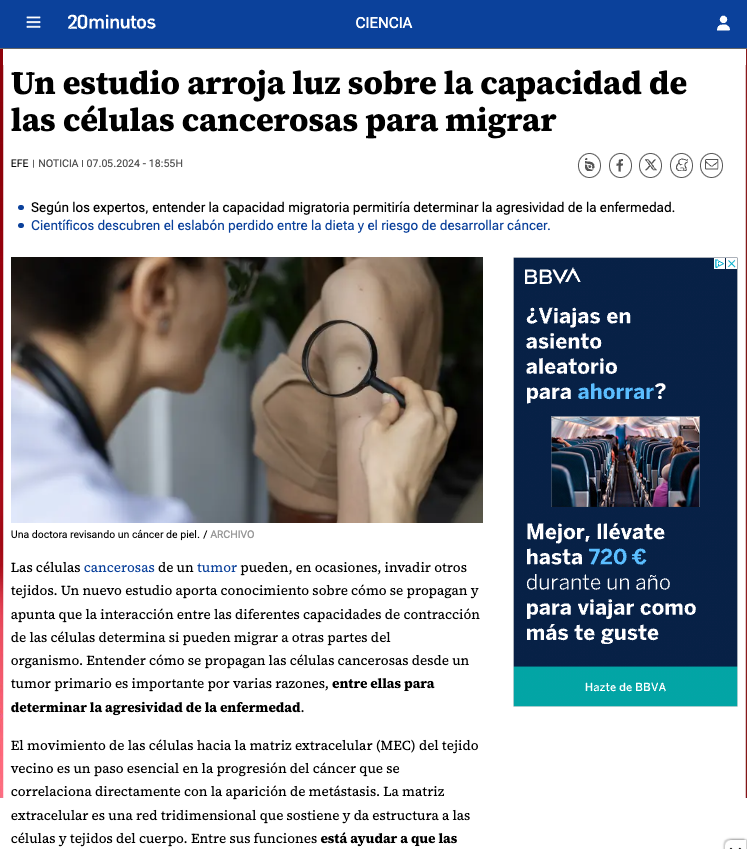
Tissues in our body can be extremely soft such as breast or brain, or very stiff such as bone. Cells in our body constantly interact mechanically with such tissues, exerting, transmitting, withstanding, and detecting forces. This mechanical interaction with the environment regulates how cells proliferate, differentiate, and move, and regulates development, tumorigenesis or wound healing.
Our research aims at unraveling – and re-engineering – the molecular mechanisms by which cells detect and respond to mechanical stimuli like forces or tissue rigidity, triggering downstream cell responses.
Just like biochemical stimuli initiate signaling cascades, mechanical forces affect the links and conformation of a network of molecules connecting cells to the extracellular matrix. This molecular and cellular response to force constitutes the phenomenon of mechanotransduction.
To study mechanotransduction, we combine biophysical techniques like magnetic and optical tweezers, Atomic Force Microscopy, traction microscopy, and microfabricated force sensors with molecular biology, advanced optical microscopy, and theoretical modelling.
Sensing the environment: Using this multi-disciplinary approach, we have unveiled a molecular mechanism that cells employ to detect and respond to the rigidity of their environment, which could be crucial in breast tissue and breast cancer (Elosegui-Artola et al., 2016 Nat. Cell Biol., and Elosegui-Artola et al. 2014, Nature Mater.). This mechanism is mediated by what is known as a “molecular clutch”: in a surprising analogy with a car engine, cells can be understood as a molecular network that can engage and disengage from its environment, just as the clutch of a car. This affects force transmission from the environment to cells, and also within different cell components. We are also expanding on the idea of the molecular clutch, to explore how cell molecular engines sense not only mechanical rigidity, but other important parameters from their environment: for instance, the composition and distribution of ligands in the extracellular matrix, or other cells. In this regard, we uncovered that this concept can explain how cells sense the spatial distribution of ligands in the extracellular matrix (Oria et al., Nature 2017). We have also demonstrated that cell-cell force transmission, mediated by a molecular clutch, is essential for cells to sense gradients in stiffness (Sunyer et al., Science 2016, in collaboration with the group of Xavier Trepat).
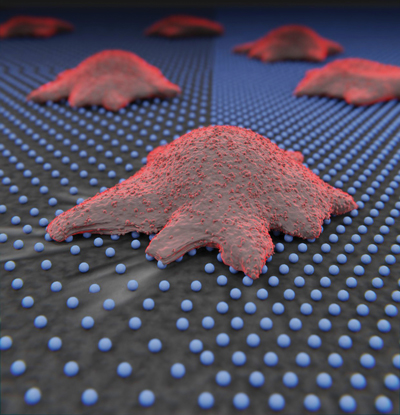
Nuclear mechanotransduction: Forces applied to cells are transmitted all the way to the cell nucleus, where they affect its function. We are studying how this force transmission affects the dynamics of transcriptional regulators, such as YAP (Elosegui-Artola et al., 2017, Cell), and how this affects cell function.
The membrane as a mechanosensor: Due to its mechanical properties, the plasma membrane itself can respond to forces and act as a mechanosensor. Recently, we have shown that cell membranes can use purely physical principles to adapt their shape in response to mechanical forces (Kosmalska et al., 2015, Nat. Commun.). We are currently studying how cells harness this physical membrane behavior to respond to signals from their environment.
Ultimately, when we determine the molecular mechanisms that communicate cells with their environment, we will understand how forces determine development when things go right, and tumor formation when they go wrong.
Video: How tissue stiffness activates cancer
STAFF
The following is a list of the current staff members of the research group:
PROJECTS
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| MECNUC · Estudio del control mecánico de la localización nuclear de proteínas (2020-2023) | MINECO Retos investigación: Proyectos I+D | Pere Roca-Cusachs |
| BLOCMEC Development of small molecules to block mechanotransduction for pancreatic cancer therapy (2021-2023) | MICIU, Proyectos Pruebas de Concepto | Pere Roca-Cusachs |
| INTROPY INhibiting mechanoTRansduction for Oncology theraPY (2021-2023) | ACCIO, Tecniospring Industry | Mamatha Nijaguna |
| INTERNATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| MECHANOCONTROL · Mechanical control of biological function (2017-2021) | European Commission, FET Proactive | Pere Roca-Cusachs |
| TALVIN · Inhibiting mechanotransduction for the treatment of pancreatic cancer (2018-2021) | European Commission, FET Innovation Launchpad | Pere Roca-Cusachs |
| MECHANOSITY Mechanical regulation of cellular behaviour in 3D viscoelastic materials (2019-2022) | European Commission, MARIE CURIE | Alberto Elosegui |
| PRIVATELY-FUNDED PROJECTS | FINANCER | PI |
|---|---|---|
| Mech4Cancer · Enabling technologies to map nuclear mechanosensing: from organoids to tumors (2020-2023) | Obra Social La Caixa Health Research Call | Pere Roca-Cusachs |
| Understanding YAP-mediated mechanotransduction in pancreatic cancer (2020-2023) | Fundació La Marató de TV3 | Pere Roca-Cusachs |
| Understanding and measuring mechanical tumor properties to improve cancer diagnosis, treatment, and survival: Application to liquid biòpsies (2017-2022) | Obra Social La Caixa | Pere Roca-Cusachs |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| Desarrollo de una terapia innovadora para el tratamiento de los tumores sólidos mediante la inhibición de la mecanotransducción (2018-2020) | MINECO, Subprograma Retos-Colaboración | Pere Roca-Cusachs |
| Understanding and measuring mechanical tumor properties to improve cancer diagnosis, treatment, and survival: Application to liquid biopsies (2017-2020) | Obra Social La Caixa | Pere Roca-Cusachs |
| IMREG El sistema acoplado entre integrinas y proteínas adaptadoras como regulador mecánico del comportamiento celular (2016-2020) | MINECO, Proyectos I+D Excelencia | Pere Roca-Cusachs |
| MECHANOMEMBRANE Redes mecanoquímicas en la membrana plasmática (2017-2018) | MINECO, Subprograma Estatal de Generación de Conocimiento “EUROPA EXCELENCIA” | Pere Roca-Cusachs |
| Stromal stiffness in tumor progression (2014-2017) | Fundació La Marató de TV3 | Pere Roca-Cusachs |
| MECBIO Red de Excelencia en Mecanobiología (2014-2016) | MINECO, Subprograma Estatal de Generación de Conocimiento «REDES DE EXCELENCIA» | Pere Roca-Cusachs |
| Inhibiting mechanostransduction as a novel therapy in the treatment of solid tumors (2017-2018) | Obra Social La Caixa | Pere Roca-Cusachs |
PUBLICATIONS
Click here for a list of publications by Pere Roca-Cusachs with IBEC affiliation.
Click here for a full list of publications including those affiliated to other organisations.
Check for more detailed information on the outputs of the Group at IBEC CRIS portal.
Publications list:
EQUIPMENT
- Confocal Microcopy
- Traction Microscopy
- Live cell fluorescence microscopy
- Cell stretching
- Cell culture
- Magnetic Tweezers
- Atomic Force Microscopy
- Surface Micro/Nano-patterning
- Optical tweezers
COLLABORATIONS
- Dr. Nils Gauthier
Mechanobiology Institute, Singapore - Prof. Miguel Ángel del Pozo
Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid - Prof. Marino Arroyo
UPC, Barcelona - Prof. Ada Cavalcanti
University of Heidelberg, Germany - Satyajit Mayor
National Centre for Biological Sciences, Bangalore, India - Sergi Garcia-Manyes
King’s College, London, UK - Louise Jones
Barts Cancer Institute, London, UK - Aránzazu del Campo
INM Saarbrücken, Germany - Patrick Derksen
UMC Utrecht, the Netherlands - Johanna Ivaska
University of Turku, Finland - Jacco van Rheenen
Netherlands Cancer Institute, Netherlands - Isaac Almendros and Ramon Farré
UB, Barcelona - Marc Martí-Renom
CNAG, Barcelona - Marc Güell
UPF, Barcelona - Francisco Real
CNIO, Madrid - Jonas Ries
Max Perutz labs, Vienna
Clinical collaborations
- University Medical Centre Utrecht
- Vall d’hebron Institute of Oncology
NEWS

El IBEC y el EMBL Barcelona coorganizan una jornada de colaboración para explorar sinergias
El Instituto de Bioingeniería de Cataluña (IBEC) y el Laboratorio Europeo de Biología Molecular (EMBL) han celebrado hoy una jornada de “matchmaking”. El evento ha reunido a investigadores e investigadoras destacadas de ambos centros con el fin de fomentar la creación de nuevas conexiones y promover el diálogo científico.

INTROPY: Un nuevo enfoque en la terapia contra el cáncer mediante la inhibición de la mecanotransducción
El investigador principal del IBEC Pere Roca-Cusachs ha obtenido una “ERC Proof of Concept Grant”. Se trata de una prestigiosa financiación que concede el Consejo Europeo de Investigación para explorar el potencial comercial y social de proyectos de investigación llevados a cabo en instituciones europeas. El proyecto de Roca-Cusachs, INTROPY, se enfoca en la inhibición de la mecanotransducción como potencial terapia contra el cáncer o la fibrosis.

Postdoctoral Researcher at the Cellular and Molecular Mechanobiology Research Group Unit
Ref: PR_PR //Deadline : 03/02/2025

El IBEC y el VHIR celebran una jornada de colaboración para fomentar las sinergias
La 1ª Jornada Colaborativa Traslacional entre el Vall d’Hebron Instituto de Investigación (VHIR) y el Instituto de Bioingeniería de Cataluña (IBEC), celebrada el 21 de noviembre, ha sido una oportunidad para conocer proyectos y líneas de investigación de ambas instituciones y promover la interacción entre los profesionales.

La viscosidad de los materiales, clave en la diferenciación celular
Un estudio liderado por el IBEC ha desvelado cómo las células madre mesenquimales responden a la viscosidad de su entorno, un aspecto fundamental en su proceso de diferenciación. Publicado en Nature Communications, esta investigación aporta nuevos conocimientos que podrían revolucionar el diseño de biomateriales para aplicaciones en medicina regenerativa.

Bioingeniería para las terapias emergentes y avanzadas en el 17º Simposio del IBEC
IBEC’s 17th Annual Symposium focused on ‘Bioengineering for Emergent and Advanced Therapies’, one of IBEC’s key application areas. Around 300 people attended the event, including local and international researchers. It was a multidisciplinary environment in which experts from other centres and the IBEC community itself had the opportunity to present their projects and share knowledge.

Pere Roca-Cusachs recibe por segunda vez la distinción del programa ICREA Academia
El investigador del IBEC Pere Roca-Cusachs ha sido galardonado con la distinción «ICREA Acadèmia» que otorga la Institución Catalana de Investigación y Estudios Avanzados (ICREA). El líder del grupo de Mecanobiología celular y molecular en el IBEC, ha recibido el premio en la categoría de Ciencias de la Vida y de la Salud.
El IBEC y el ICMS se reencuentran en su Simposio Anual en colaboración
Hoy, 14 de marzo, se ha celebrado el simposio conjunto ICMS-IBEC. Un evento coorganizado por el IBEC y el Instituto de Sistemas Moleculares Complejos (ICMS). Durante la jornada, investigadores del IBEC y del ICMS han compartido sus áreas de investigación, buscando fortalecer los lazos científicos entre ambas instituciones.

BIST Forum, un encuentro para poner en valor la investigación de frontera
El BIST Forum ha tratado de cómo la ciencia de excelencia potencia el desarrollo de la sociedad y el crecimiento económico. Han asistido el presidente de la Generalitat, el alcalde de Barcelona, los responsables de las máximas instituciones económicas y los rectores de las principales universidades. En el acto se han anunciado los nuevos proyectos BIST IGNITE para la investigación multidisciplinar, de los cuales tres cuentan con la participación del IBEC.
JOBS
Laboratory Technician at the Cellular and Molecular Mechanobiology Research Group
Ref: LT_PR // Deadline: 12/10/2025
Research Assistant position at the Cellular and Molecular Mechanobiology Research Group
Ref: RA-PR // Deadline: 27/06/2025
Research Assistant at the Cellular and Molecular Mechanobiology Research Group
Ref: RA-PR // Deadline: 18/10/2024
Postdoctoral Researcher at the Cellular and Molecular Mechanobiology Research Group
Ref: PR_PR // Deadline: 13/03/2024
Laboratory Technician at the Cellular and Molecular Mechanobiology Research Group (LT_PR)
Ref: LT_PR/Deadline: 20/02/2024
Postdoctoral researcher at the Cellular and Molecular Mechanobiology Research Group.
Ref: PR-PR //Deadline: 15/12/2023
Postdoctoral researcher at the Cellular and Molecular Mechanobiology Research Group
Ref: PR_PR/Deadline:20/11/2023
Research Assistant at the Cellular and Molecular Mechanobiology Research Group
Ref: RA_PR/Deadline:07/11/2023
Research Assistant at the Cellular and Molecular Mechanobiology Research Group
Ref: RA_PR/ Deadline: 16/10/2023


 ibecbarcelona.eu
ibecbarcelona.eu