About
Currently, a myriad of drug carriers or delivery systems have been developped to improve the overall performance and safety of therapautic agents. However, our ability to treat neurological maladies, genetic syndromes, cancer, etc., still remains a major challenge. One of the prime obstacles is our limited knowledge on the biological parameters that regulate the interaction of these systems with our tissues and, hence, our inability to gain non-invasive, efficient, and specific access within the body, its cells, and subcellular organelles.
To generate knowledge and tools to overcome this obstacle, we study the biological mechanisms ruling how our cells and tissues transport cargoes to precise destinations within our bodies, and apply this knowledge to the design of nanodevices for improved delivery of therapeutic agents to specific disease sites.
Biologically-Controlled Transport of Drug Carriers
Most current strategies aim to bind a drug nanocarrier to a specific cell-surface receptor; but after said binding takes place, receptor-associated signaling and transport processes take control. Instead, our laboratory can impart a drug carrier control over the biological events that occur beyond binding. We have shown how using the same receptor, the kinetics, mechanism, and destination of a drug carrier can be modulated by: (a) varying its size, shape, and targeting valency; (b) varying the receptor epitope to which the carrier binds; (c) coupling carriers to signaling molecules; (d) combining targeting to several receptors; or (e) coupling targeting moieties with anti-phagocytic moieties on the surface of drug carriers.
Transport of Drug Carriers Across Physiological Barriers
Crossing the linings that separate body and cellular compartments is paramount for efficient drug delivery. We were first to identify a natural pathway regulated by ICAM-1, a cell adhesion molecule overexpressed by most cells under pathological states, which enables transcytosis across the epithelial barrier that separates the gastrointestinal tract from the bloodstream for oral delivery, and the blood-brain barrier that separates the bloodstream from the brain tissue for delivery of therapeutics against neurological diseases. We also work with DNA-built nanocarriers which enable uptake within cells and endosomal escape for delivery to the cytosol and other subcellular compartments.
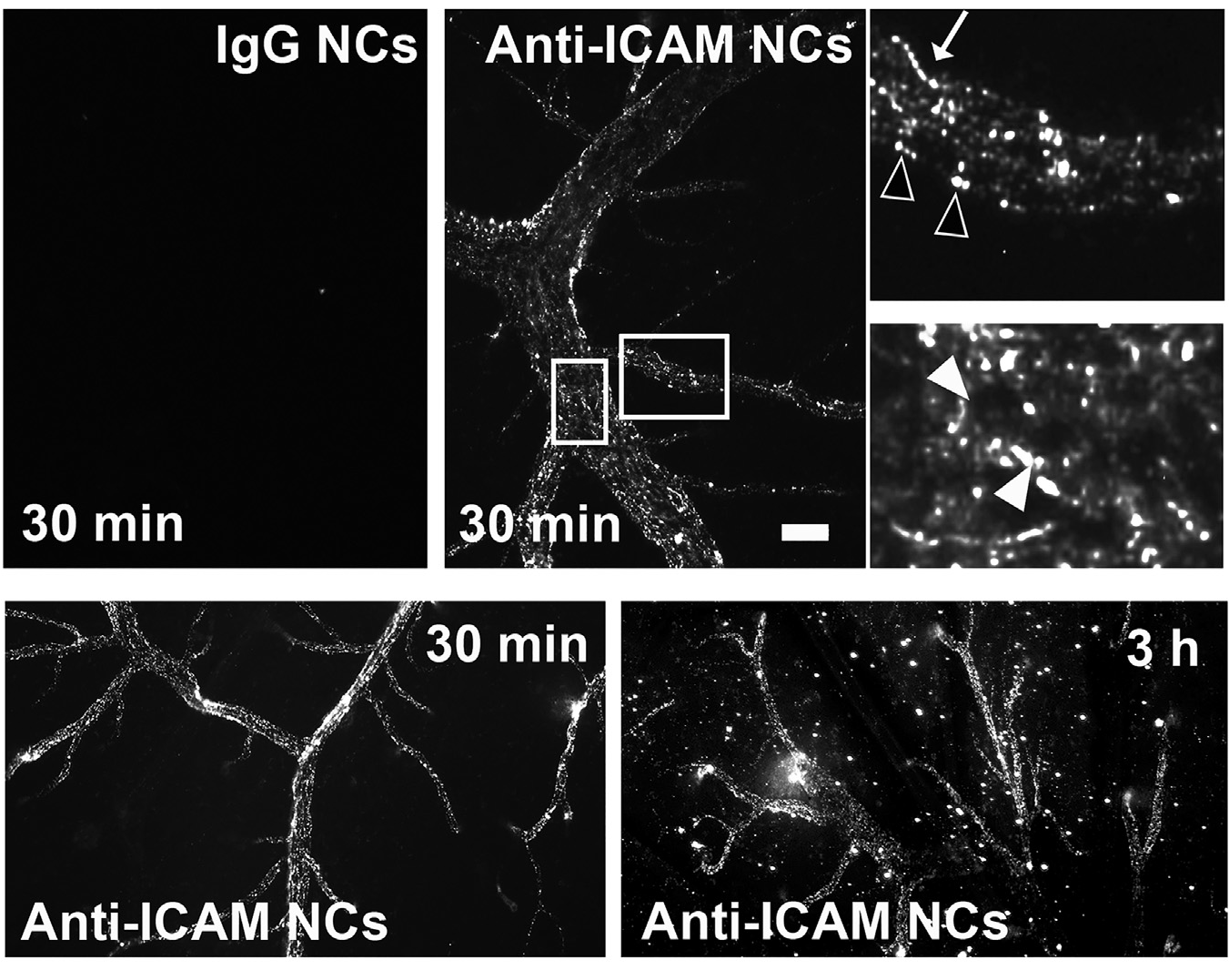
Figure 1. ICAM-1-targeted nanocarriers (anti-ICAM NCs) crossing the blood-brain barrier in an animal model. Fluorescence microscopy of cortical brain samples isolated from mice 30 min or 3 h after intravenous injection with green-fluorescent anti-ICAM NCs or control IgG NCs. Scale bar = 10 μm. Boxes = regions magnified 3-fold in the right panels. Arrows = NCs on the blood-facing surface of the blood-brain barrier. Open arrowheads = NCs on the brain-facing surface of the blood-brain barrier. Closed arrowheads = NCs inside of brain endothelial cells. Extracted from Manthe et al., Intertwined mechanisms define transport of anti-ICAM nanocarriers across the endothelium and brain delivery of a therapeutic enzyme. J Control Rel. 324 (2020):181-193).
Improving Treatment of Lysosomal Disorders
Pathologies due to monogenic deficiencies, such as the case of lysosomal disorders, are valuable models to study disease progression and therapeutic intervention because of their well-known etiology, unequivocal diagnosis, and availability regarding patient samples, diverse cell types, and animal models. Since these diseases associate to either acute or long-term effects depending on genetic severity, and they associate with neurodegeneration, cardiovascular, metabolic, and cancer-like syndromes, they represent excellent disease models. Consequently, we are applying targeted nanotechnology concepts to the treatment of genetic lysosomal disorders. Current therapies by i.v. enzyme infusion are only helpful for diseases where clearance cells and organs (liver, spleen, macrophages, etc.) are the main targets. Yet, delivery to other organs such as the brain, lungs, etc. hinders treatment for most of these diseases. Using types A and B Niemann-Pick, Fabry, and Gaucher diseases as examples, we are developing new therapeutic strategies to deliver therapeutic enzymes to all affected organs in animal models, which holds considerable translational potential.
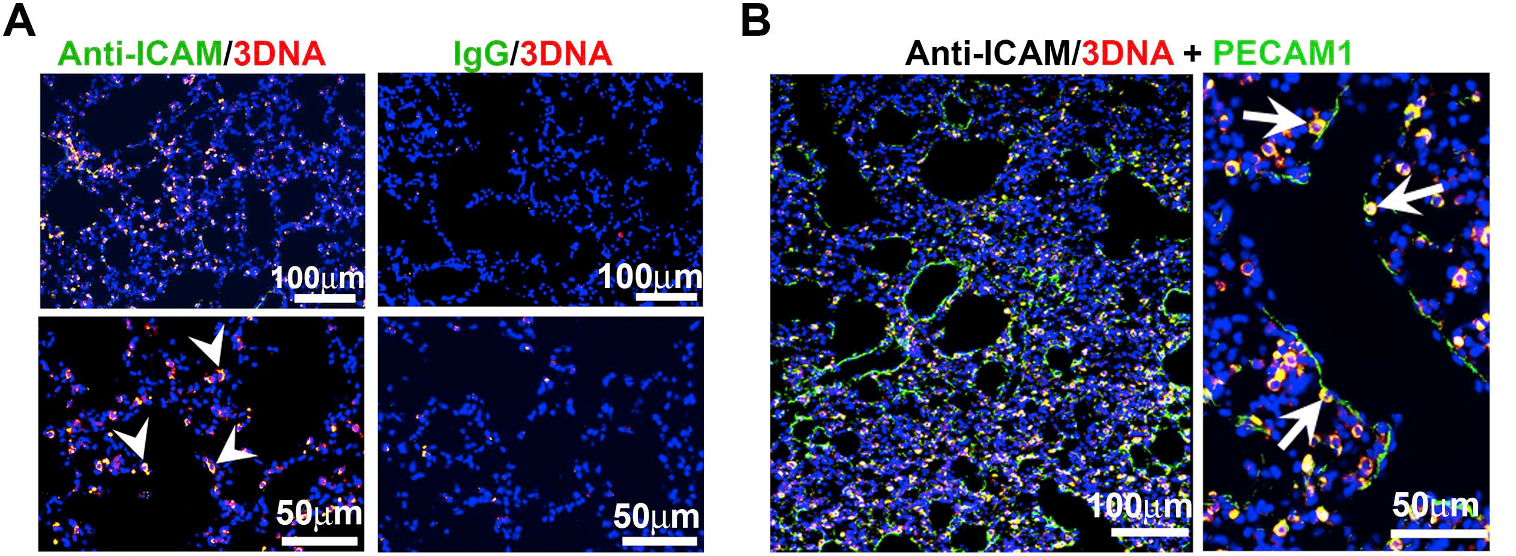
Figure 2. ICAM-1 targeted DNA-made nanocarriers (anti-ICAM/3DNA) for drug delivery to the lungs. Cy3-labeled 3DNA targeted to ICAM-1 (anti-ICAM/Cy3-3DNA) or non-targeted controls (IgG/Cy3-3DNA) were intravenously injected in mice. Lungs were isolated and processed at sacrifice at 60 min. (A) Confocal microscopy shows Cy3 3DNA (red), while the targeting coat (antibody) is visualized using a FITC-secondary antibody (green). Arrowheads show colocalization of the antibody on the coat of 3DNA. (B) Confocal microscopy showing anti-ICAM/Cy3-3DNA (red) and lung endothelial cells visualized using polyclonal anti-PECAM-1+FITC-secondary antibody (green). Arrows indicate colocalization of Cy3-3DNA with endothelial PECAM-1. (Extracted from Roki et al, Unprecedently high targeting specific to lung ICAM-1 using 3DNA nanocarriers. J Control Release. 305 (2019):41-49).
Staff
Silvia Muro
Projects
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| CROSSTARGET · Desarrollo de nuevas herramientas traslacionales multi-especie para el direccionamiento de terapias con precisión de órgano y subcelular (2019 – 2022) | Ministerio de Ciencia, Innovación y Universidades | Silvia Muro |
| NANO-GBA · Assessing the effects of glucocerebrosidase (GBA) alterations on receptor membrane nanoarchitecture to design improved nanomedicines(2020 – 2021) | BIST Ignite Program | Silvia Muro |
| FUNDRAISING PROJECTS | FINANCER | PI |
|---|---|---|
| Campaña FasterFuture «A por el Parkinson» (2019 – 2021) | Fundraising | Silvia Muro |
| FINISHED PROJECTS | FINANCER | PI |
|---|---|---|
| BBB2GATE · Control diferencial del transporte de vehiculos terapeuticos dentro versus a traves de la barrera hematoencefalica (2018 – 2020) | MINECO | Silvia Muro |
Publications
Equipment
- Dynamic Light Scattering & ζ-Potential: Malvern’s Zetasizer Ultra.
Determination of the size, concentration, mobility, ζ-potential of nanoparticles, including high resolution size and MW distribution, non-invasive retrodispersion optics for wide concentration ranges, a fluorescent filter wheel to minimize artifacts from fluorescent samples, multiangle scattering (back, side, and forward scatter) to determine particle concentration without calibration, and constant current mode to measure high conductivity simples. - Gel Permeation Chromatography: Malvern’s OMNISEC.
Detector system (refraction index) of 640 nm y 50 mW, as well low (<10°) angle for highly sensitive determination of the absolute MW of polymers and proteins, upgradable to measure viscosity, branching, etc. Chromatography module, regulable pump, degasifier, sampler adaptable to many sample formats, temperature control for high MW polymers, columns/pre-columns for separation of polymers and proteins, and fraction collector. - Confocal-epifluorescence microscope: Zeiss LSM 700 microscope with 100 mW max output, 400-650 wavelength range, and IIIb class laser; 10x: EC Plan-NEOFLUAR, 20x: LD Plan-NEOFLUAR, 50x: EC Epiplan-NEOFLUAR, and 100x/1.3 oil: Plan-NEOFLUAR objectives; AxioCam Mrm camera; XLmulti S1 incubator system, and Zen 2 blue software.
Collaborations
- Dr. Alexander Andrianov, University of Maryland, MD, USA.
- Dr. Yu Chen, University of Maryland College Park, MD, USA.
- Dr. Mandy Esch, National Institutes for Standards and Technology, Gaithersburg, MD, USA.
- Dr. Robert Getts, Genisphere LLC, Hatfield, PA, USA.
- Dr. Hamid Ghandehari, University of Utah, UT, USA.
- Dr. Janet Hoenicka, Sant Joan de Deu Hospital, Barcelona, Spain.
- Dr. Christopher Jewell, University of Maryland College Park, MD, USA.
- Dr. Joe Kao, University of Maryland Baltimore, MD, USA.
- Dr. Peter Kofinas, University of Maryland College Park, MD, USA.
- Dr. Juan Marugan and Dr. Wei Zheng, National Institutes of Health, Rockville, MD, USA.
- Dr. Vladimir Muzykantov, University of Pennsylvania, Philadelphia, PA, USA.
- Dr. Gianfranco Pasut, University of Padova, Padova, Italy.
- Dr. Edward Schuchman, Mount Sinai School of Medicine, New York, NY, USA.
- Dr. Brigitte Stadler, Aarhus University, Denmark.
- Dr. Maria Jesus Vicent, Principe Felipe Research Center, Valencia, Spain.
- Dr. Alberto Fernández de las Nieves, ICREA & University of Barcelona, Spain.
- Dr. Xavier Rodó, ICREA & ISGlobal, Barcelona, Spain.
- Dr. Antonio Alcamí, CNB & CSIC, Madrid, Spain.
- Dr. Dolores Ledesma, CBM & CSIC, Madrid, Spain.
- Dr. Ricardo Feldman, University of Maryland, Baltimore, MD, USA.
- Dr. Ramón Farré, Clinic Hospital & University of Barcelona, Spain
- Dr. Jesús Villarrubia, Ramón y Cajal Hospital, Madrid, Spain
News
Identifican las mejores vías para llevar fármacos al cerebro durante la enfermedad de Niemann-Pick tipo A, una patología neurológica
Investigadores identifican como la enfermedad neurológica Niemann-Pick tipo A altera las vías naturales de entrada de moléculas al cerebro e identificaron las más activas y propensas a ser utilizadas con … Read more
La Asociación Catalana para el Parkinson visita IBEC
El pasado día 18 de noviembre, miembros de la Asociación Catalana para el Parkinson visitaron el Instituto de Bioingeniería de Catalunya (IBEC) para conocer, de primera mano, el trabajo de Silvia Muro y su grupo de investigación, los cuales utilizan la nanotecnología para tratar de hallar soluciones a esta enfermedad. El trabajo de la profesora Muro ha contado, entre otros, con el apoyo de las donaciones recibidas a través del programa “Faster Future” del IBEC.
La campaña Faster Future contra el Parkinson culmina con nuevos avances científicos
El programa IBEC Faster Future ha hecho posible, durante los dos últimos años, y gracias a las aportaciones de los donantes, el estudio de anticuerpos como base de un producto terapéutico para el tratamiento del Parkinson. Los investigadores presentan ahora los avances científicos, y seguirán trabajando para tratar de dar respuesta a unos de los mayores desafíos de la medicina moderna: las patologías neurodegenerativas como el Parkinson.
Silvia Muro, líder de grupo en IBEC, elegida para unirse a la élite estadounidense de ingeniería médica y biológica
El Instituto Americano de Ingeniería Médica y Biológica (AIMBE) ha anunciado la elección de la Dra. Silvia Muro como miembro del College of Fellows por sus logros en el comportamiento de las nanomedicinas a nivel celular y molecular.
Investigadores del IBEC hallan una nueva forma de transportar eficazmente fármacos al cerebro
Un grupo internacional de investigadores liderados por la profesora ICREA Silvia Muro del Instituto de Bioingeniería de Catalunya (IBEC) y la Universidad de Maryland (Estados Unidos) ha identificado una nueva forma de transportar fármacos al cerebro, uno de los grandes desafíos de la ciencia farmacéutica actual, lo que podría ayudar a diseñar nuevos tratamientos para enfermedades neurológicas tales como el Parkinson o el Alzheimer. Para la elaboración del trabajo, publicado esta semana en la prestigiosa revista Journal of Controlled Release, los expertos unieron un anticuerpo capaz de reconocer la proteína ICAM-1 –una molécula expresada en la superficie de los vasos sanguíneos- a una serie de nanopartículas poliméricas que pueden transportar un fármaco e inyectarlo por vía intravenosa.
El IBEC dedica su segunda edición del programa Faster Future a la lucha contra el Parkinson
El programa IBEC Faster Future, que persigue recaudar fondos para llevar a la práctica clínica determinados proyectos que ya se encuentran en una etapa avanzada de investigación, hará posible el desarrollo de un nuevo anticuerpo que será la base de un producto terapéutico para el tratamiento del Parkinson. La campaña del programa Faster Future “A por el Parkinson”, que se lanza hoy y que permanecerá abierta hasta el 30 de abril, tiene por objetivo recaudar los 50.000€ necesarios para acelerar esta investigación, esperando obtener resultados favorables en un plazo de año y medio.
IBEC project to defy blood-brain barrier awarded in highly competitive call
IBEC group leader Silvia Muro has been granted funding in MINECO’s ‘Explora Ciencia’ and ‘Explora Tecnología’ 2017 call. It’s the first competitive grant for Silvia and her group since she joined IBEC at the end of 2017, and one of only 97 research projects to be financed out of the 1594 applications submitted – a success rate of only 6%. The project, ‘Controlling the differential transport of therapeutic cargoes into versus across the BBB (BBB2GATE)’ will aim to develop drug vehicles that can cross the blood-brain barrier using the natural routes that the body’s substances use to circumvent this obstruction.
Jobs
Research Assistant at the Targeted Therapeutics & Nanodevices Research Group
Application Deadline: 02/12/2021Ref: RA-SM The Targeted Therapeutics and nanodevices group at the Institute for Bioengineering of Catalonia (IBEC) is looking for a Research Assistant to collaborate in a research project focused on drug delivery systems capable of targeting and crossing the blood-brain barrier (BBB) for treatment of neurological diseases involving lysosomal alterations and/or inflammation.


 ibecbarcelona.eu
ibecbarcelona.eu