About
We are chemists, physicists, mathematicians, engineers, biologists who work alongside to design bionic units that mimic specific biological functions and/or introduce operations that do not exist in Nature. We apply a constructionist approach where we mimic biological complexity in the form of design principles to produce functional units from simple building blocks and their interactions. We called such an approach: Molecular Bionics.
We are engaged in several activities involving the synthesis and characterisation of novel hierarchal materials whose properties are the result of the holistic combination of its components:
Molecular Engineering
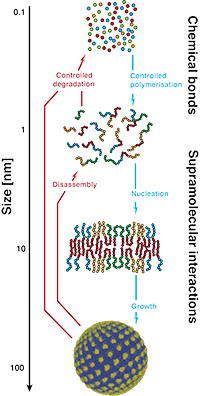
We combine synthetic and supramolecular chemistry to tune inter/intramolecular interactions and self-assembly processes to form dynamic soft materials whose molecular, supramolecular and mesoscale structures are tuned and fit for the final application (pictured right: molecular engineering of nanoscopic structures starting from molecule passing to polymers and finally to supra molecular structures).
Physical Biology
Our materials are designed to interact with living systems and thus its biological activity is studied in high detail. We have developed and established new methodologies to study living systems and how synthetic materials interact with them combining holistically physical and life sciences (Physical Biology).
Synthetic Biology
Both know-hows are applied to study biological organisation and complexity creating synthetic surrogates that act as models, as well as to engineer novel sophisticated ways to interact with living organisms.
Somanautics
In analogy to medical bionics, where engineering and physical science converge to the design of replacement and/or enhancement of malfunctioning body parts, we take inspiration from viruses, trafficking vesicles and exosomes to apply molecular engineering to create nanoscopic carriers that can navigate the human body (Somanautics) with the final aim to improve drug delivery or create new diagnostic tools.
Visit our external website to find out more.

Staff
Giuseppe Battaglia
Projects
| INTERNATIONAL GRANTS | FINANCER | PI |
|---|---|---|
| CheSSTag · Chemotactic Super-Selective Targeting of Gliomas (2020-2023) | European Comission / ERC-CoG | G. Battaglia |
| NATIONAL PROJECTS | FINANCER | PI |
|---|---|---|
| BrainPePN · Nanomedicinas de precisión que penetran el cerebro (2021-2024) | MICIU / Retos investigación: Proyectos I+D | G. Battaglia |
| FUNDRAISING PROJECTS | FINANCER | PI |
|---|---|---|
| A por la COVID-19 (2021-2022) | IBEC / Faster Future 2020 | G. Battaglia |
Publications
Equipment
- State-of-the-art facilities for cell culture including 5 class A cell cabinets: one dedicated for LPS and RNAse free cell culture and one dedicated for infected tissues
- Fluorescence Activated Cell Sorting (FACS)
- Confocal microscope to perform live cell 4D imaging
- Thermocycler
- Real-time PCR
- Automated Western Blot
- Gel Permeation Chromatography
- High-Performance Liquid Chromatography
- Ultra Performance Liquid Chromatography equipped with fluorescence, UV/Vis and Infrared and light scattering detectors
- Dynamic light scattering unit
- Nanoparticle tracking analysis
- UV and Fluorescence spectroscopy
- Automated liquid handling units
- Nanoparticle production units
Collaborations
- Xavier Salvatella
IRB Barcelona - Francesca Peiro
Physics-University of Barcelona - Kostas Kostarellos
Life Science- University of Manchester/ICN2 - Giorgio Volpe
Chemistry-UCL - Simona Parrinello
Cancer Institute -UCL - Finn Werner
Structural Biology -UCL - Nick Lane
Evolutionary Biology -UCL - Darren Hargraves
Pediatric Neuro-Oncology -UCL - Timothy McHugh
Clinical Microbiology =UCL - Sebastian Brander
Neurology -UCL - Joan Abbott
Physiology -King’s College London - Molly Stevens
Bioengineering -Imperial College London - Stefano Angioletti-Uberti
Materials Science -Imperial College London - Ricardo Sapienza
Physics -Imperial College London - Daan Frenkel
Chemisty-University of Cambridge - Charlotte Williams
Chemistry -University of Oxford - Francesco Gervasio
Pharmacology -University of Geneve/UCL, UK - Francesco Stellacci
Bionegineering -EPFL Switzerland - Tambet Tessalu
Cancer Biology -University of Tartu (Estonia)/ Sanford Burnham Prebys Medical Discovery Institute - Darrel Irvine
Bioengineering -MIT - Xiaohe Tian
Life Sciences University of Anhui - Yupeng Tian
Chemistry University of Anhui - Lei Luo
Pharmacy -Southwest University, China - Kai Luo
HuaXi hospital Sichuan University - Darren Hargrave
Great Ormond Street Hospital, UCLH London - Sebastian Brander
Queen Square National Centre for Neurology, UCLH London
News
IBEC and ICMS reunite once more at their Annual Collaborative Symposium
Today, on March 14th, the joint ICMS-IBEC symposium took place. This event was co-organized by IBEC and the Institute for Complex Molecular Systems (ICMS). Throughout the conference, researchers from both IBEC and ICMS presented their areas of research, aiming to enhance the scientific collaboration between the two institutions.
BIST Forum, a meeting to highlight the value of frontier research
The BIST Forum has discussed how excellent science enhances the development of society and economic growth. The event was attended by the President of the Catalan Government, the Mayor of Barcelona, the heads of the highest economic institutions and the rectors of the main universities. At the event, the new BIST IGNITE projects for multidisciplinary research were announced, three of which have the participation of IBEC.
European funding to take anti-inflammatory nanomedicines from the lab to society
IBEC researcher Giuseppe Battaglia has received an ERC Proof of Concept Grant. It is a prestigious funding awarded by the European Research Council to explore the commercial and societal potential … Read more
Animal Lab technician
Application Deadline: 30/06/2022Ref: LT_GB The Molecular Bionics group at the Institute for Bioengineering of Catalonia (IBEC) led by Prof. Giuseppe Battaglia at the Institute for Bioengineering of Catalonia (IBEC) is looking for a laboratory technician to support the animal research lines of the biology section of the team, as well as to contribute to the general lab maintenance.
Three IBEC researchers granted with “La Caixa Foundation” fellowships for young scientific leaders
Veronika Magdanz, of the Nano Intelligent Devices Group led by Samuel Sánchez, as well as Iris Batalha and Mohit Kumar of the Molecular Bionics group led by Giuseppe Battaglia, have … Read more
IBEC researchers participate in the “European Researchers’ Night”
Last Friday, September 24, the “European Researchers’ Night” took place, an event that is held on a European scale in more than 300 cities in 30 different countries. The objective of this event is to publicize the diversity of science and its impact on the daily lives of citizens in a close and inspiring way. For yet another year, IBEC has not wanted to miss it and has been present at various activities.
A mechanism to cross the blood-brain bareer has been discovered
Giuseppe Battaglia, leader of the IBEC “Molecular Bionics” group and ICREA Research Professor appears in various media for his recent study describing a mechanism and conditions that allow molecules to efficiently cross the blood-brain barrier, the protective layer of the brain.
IBEC calls society to action to accelerate research against COVID19
The Institute for Bioengineering of Catalonia (IBEC) launches the Faster Future “A por la COVID19” campaign, with the aim of raising the 100.000€ needed to accelerate three research projects in collaboration with hospitals and patients associations.
Researchers discover a mechanism to cross the protective barrier of the brain
An international study led by IBEC researcher Giuseppe Battaglia identifies a mechanism and conditions that allow molecules to efficiently cross the blood-brain barrier, the protective layer of the brain. This study describes the role of protein LRP1, bringing light to safe and efficient entrance of drugs to the brain.
Jobs
Senior Laboratory Technician at the Molecular Bionics Research Group (SLT_GB)
Ref: SLT_GB // Deadline: 24/12/2023
Research Assistant at the Molecular Bionics Research Group (RA_GB)
Ref: RA_GB // Deadline 12/12/2023
Part-time lab manager (Tissue Engineering) in the Molecular Bionics Group (LM_GB)
Ref: LM_GB // Deadline: 31/08/2023
Senior Translational Material Scientist/Chemist at the Molecular Bionics Group (STM_GB)
Ref: STM_GB // Deadline: 31/08/2023
Predoctoral researcher at the Molecular Bionics Research Group
Ref.: PhD-GB // Deadline: 18/02/2023


 ibecbarcelona.eu
ibecbarcelona.eu